miR-148a-3p inhibits the proliferation and migration of bladder cancer via regulating the expression of ROCK-1
- Published
- Accepted
- Received
- Academic Editor
- Alberto Davalos
- Subject Areas
- Cell Biology, Molecular Biology, Oncology, Urology
- Keywords
- Bladder cancer, Biological behavior, miRNA, miR-148a-3p
- Copyright
- © 2022 Xu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. miR-148a-3p inhibits the proliferation and migration of bladder cancer via regulating the expression of ROCK-1. PeerJ 10:e12724 https://doi.org/10.7717/peerj.12724
Abstract
Purpose
To investigate the mechanism of miR-148a-3p regulating the proliferation and migration of bladder tumor cells.
Materials and Methods
We conducted a preliminary study to detect the relative expression of miR-148a-3p in bladder cancer and para-cancerous tissue samples. Three bladder tumor cell lines, T24, 5,637 and UM-UC-3, were selected. The expression levels of miR-148a-3p were artificially regulated with miR-148a-3p mimics and the miR-148a-3p inhibitor. The relative expression levels of miR-148a-3p in the samples of each cell line were determined. Cell Counting Kit-8 (CCK-8) was used to detect cell proliferation, while the effect of the miR-148a-3p mimics and inhibitor on tumor cell migration was detected by wound healing assay. Flow cytometry assay was carried out to explore the effect of miR-148a-3p on cell apoptosis. Dual-luciferase reporter assay was performed in order to verify miR-148a-3p’s target gene. The expressions of ROCK-1 and Bcl-2 were analyzed by western blot.
Results
The relative expression of miR-148a-3p in tumor and adjacent tissues was assessed with qRT-PCR (P < 0.05) and found to be significantly lower in the tumor tissues than the adjacent tissues. The data obtained from the CCK-8 and wound healing assay showed that intracellular transfection of miR-148a-3p mimics could inhibit cell proliferation and migration, while the miR-148a-3p inhibitor promoted them. Overexpression of miR-148a-3p promoted cell apoptosis in the T24 and 5,637 cell lines. The dual-luciferase reporter assay verified that ROCK-1 is a direct target of miR-148a-3p. Western blot showed that miR-148a-3p overexpression downregulated the expression of ROCK-1 and Bcl-2, while miR-148a-3p knockdown upregulated the expression of ROCK-1 and Bcl-2.
Conclusions
We confirmed that miR-148a-3p was significantly decreased in bladder cancer cells. miR-148a-3p overexpression inhibited bladder cancer cell proliferation and migration, whereas miR-148a-3p knockdown promoted bladder cancer cell proliferation and migration. Moreover, we found that ROCK-1 was a downstream target of miR-148a-3p. We also found that miR-148a-3p induced cell apoptosis by regulating the expression of Bcl-2. However, the deeper mechanism of this regulatory relationship needs further study.
Introduction
Bladder cancer is common worldwide and is the most frequently occurring malignancy in the urinary system (Cumberbatch et al., 2018; Grayson, 2017). Less than 62% of patients survive 5 years (Choueiri & Raghavan, 2008). There is an upward trend in the morbidity and detection rate of bladder cancer (Antoni et al., 2017; Tuccori et al., 2016). Therefore, identifying the pathogenic mechanism of bladder cancer is crucial for improving patients’ prediction of prognosis and survival time.
MicroRNAs (miRNAs/miRs) are small (18–24 nucleotides in length), endogenous non-coding RNA, which comprise a class of target genes that regulate their expression by promoting degradations or blocking translations of their target mRNAs (Bartel, 2004; Bartel, 2009). Emerging evidence has shown that miRNAs are associated with various tumors, including bladder cancer (Calin & Croce, 2006; Wang et al., 2016). They can affect cell differentiation, proliferation, and apoptosis by regulating many oncogenes and tumor suppressors depending on context and targets (Wang et al., 2010). miR-148a was shown to suppress the epithelial to mesenchymal transition in non-small cell lung cancer and reduce metastasis in gastric cancer by targeting ROCK-1 (Li et al., 2013; Zheng et al., 2011). In pancreatic and colorectal cancers, miR-148a has been shown to induce apoptosis by targeting Bcl-2 (Zhang et al., 2011; Zhang et al., 2014).
In previous studies, our research team demonstrated that miR-148a-3p expression was decreased in bladder cancer specimens and that reduced miR-148a-3p expression was associated with shorter survival time (Ma et al., 2016). However, the specific effects of miR-148a-3p on the biological processes of bladder cancer cells are not well elucidated. Therefore, this study was performed to investigate the relationship between miR-148a-3p and the proliferation and migration of bladder tumor cells and to explore the specific mechanism of how miR-148a-3p regulates the biological behavior of bladder tumor cells.
Materials and Methods
To verify the quantification of miR-148a-3p expression in the bladder cancer and cancer adjacent tissues, we collected 10 bladder cancer and cancer adjacent tissue samples in our preliminary study. All the specimens were enclosed in liquid nitrogen immediately after resection during the surgery. All patients signed the informed consent and this study was approved by the Institutional Review Board of Qilu hospital of Shandong University (No. 2020045).
Cell culture
T24, 5,637, and UM-UC-3 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The T24 and 5,637 cell lines were cultured in RPMI 1,640 while the UM-UC-3 cell line was cultured in DMEM. The culture medium was supplemented with 10% fetal bovine serum (FBS, BI, Israel). All the cell lines were cultured in a 37 °C incubator with 5% CO2. The culture solution did not contain antibiotics, however 0.25% trypsin solution was added to it. The culture solution was replaced every 2 days and cell passage was performed every 4 days.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cells using the Trizol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was generated using the miRNA RT Kit (Takara, Japan). According to the instruction book, the reverse transcription conditions were set as follows: 37 °C for 60 min and 85 °C for 5 min. Quantitative RT-PCR was carried out on the LightCycler 480 (Roche, Basel, CH, USA) with the fluorescence quantitative RT-PCR Kit (TB Green, Takara, Japan). U6 was used as an endogenous control for normalization of the data. miR-148a-3p forward primer: TCAGTGCACTACAGAACTTTG, U6 primer: forward, 5′‑AAA GCA AAT CAT CGG ACG ACC‑3′, reverse, 5′‑GTA CAA CAC ATT GTT TCC TCG GA‑3′. All RT-PCRs were performed in triplicate, and the data are presented as the mean ± standard deviation (SD).
Cell transfection
To verify the influence of miR-148a-3p on the cell biological behaviors such as growth, migration, and proliferation of bladder cancer, we used miR-148a-3p mimics and the miR-148a-3p inhibitor for the manual alteration of miR-148a-3p expression. The miR-148a-3p mimics (sense: 5’-UCAGUGCACUACAGAACUUUGU-3’, antisense: 5’-AAAGUUCUGUAGUGCACUGAUU-3’), negative control (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′), miR-148a-3p inhibitor (5′-ACAAAGUUCUGUAGUGCACUGA-3′) and inhibitor NC (5′-CAGUACUUUUGUGUAGUACAA -3′) (GenePharma, Shanghai, China) were transfected into T24, 5,637, and UM-UC-3 cells with Lipofectamine 3,000 reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s protocol. In brief, cells were cultured in 6-well plates for cell planking. The day before transfection, an appropriate number of cells were inoculated into each well in 2 ml antibiotic-free medium, so that the cells could reach 60–70% confluence the next day. The T24, 5,637, and UM-UC-3 cells were transfected using Lipofectamine 3,000 reagent in serum-free Opti-MEM medium according to the manufacturer’s instructions. Transfection concentration of mimics was 80 nmol/L while the inhibitor was 100 nmol/L. The solution was changed 6 h after adding the transfection reagent into the medium.
Cell Counting Kit-8 (CCK-8) assay
Cell Counting Kit-8 (CCK-8, Bioss, China) was used to analyze the cell proliferation of bladder cancer. After transfection for 24 h, the T24 and 5,637 cells were inoculated into a 96‑well plate after digestion by trypsin with 2000 cells in each well. The cells were treated with 10 μl of CCK-8 solution into each well after culturing for 0, 24, 48, 72, and 96 h. Subsequently, the culture plate was incubated in the dark for 1 h and a microplate reader (Tecan, Männedorf, Switzerland) was used to measure the absorbance at 450 nm to detect the condition of cell proliferation. This process was repeated 3 times.
Cell apoptosis analysis
T24 and 5,637 cells were transfected with miR-148a-3p mimics and NC using Lipofectamine 3,000. After 72 h, cells were harvested and stained with Annexin V-FITC and PI (KeyGen, BioTECH, China). Flow cytometry assay was carried out on a FACS Calibur FlowCytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Wound healing assay
The above transfection scheme was used to transfect T24 cells. After waiting 24 h for the complete adherence of cells, a scratch was performed in each well with the pipette head of 200 ul; each scratch was observed for healing and photographed with a microscope at 0 h and 12 h after the scratch.
Western blotting
The T24 and 5,637 cell lines were transfected with miR-148a-3p mimics, the miR-148a-3p inhibitor, negative control, and inhibitor NC using the methods above. 48 h after transfection, 1ml of RIPA buffer (Beyotime, China), 10 ul of protease inhibitor, and 10ul of phosphorylase inhibitor were used for total protein extraction and determination of protein density. The efficacy of transfection was tested by western blotting. The primary antibodies were as follow: anti-ROCK-1 antibody (1:1,000, Cell Signaling Technology, Danvers, MA, USA) and anti-Bcl-2 antibody (1:1,000, Cell Signaling Technology, Danvers, MA, USA). GAPDH was used as an endogenous control for normalization of the data.
Dual-luciferase reporter assay
Dual-luciferase reporter assay was performed in order to verify miR-148a-3p’s target gene. The pmirGLO luciferase vector (GeneCreat, China) containing the wide-type ROCK-1 3′UTR region and pmirGLO luciferase vector containing the mutant-type ROCK-1 3′UTR region were transfected with miR-148a-3p mimics or its control (mimic-NC) using Lipofectamine 3,000 according to the manufacturer’s instruction. Firefly and Renilla luciferase activities were measured by the dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) after 24 h.
Statistical analysis
Statistical analysis was performed with the SPSS 18.0 (SPSS Inc., Chicago, IL, USA) software system and the GraphPad Prism 5 software (GraphPad Software Inc, San Diego, CA, USA). The measurement data were shown as a mean ± SD and a t-test or an F-test was used for comparisons between groups. P < 0.05 was considered statistically significant.
Results
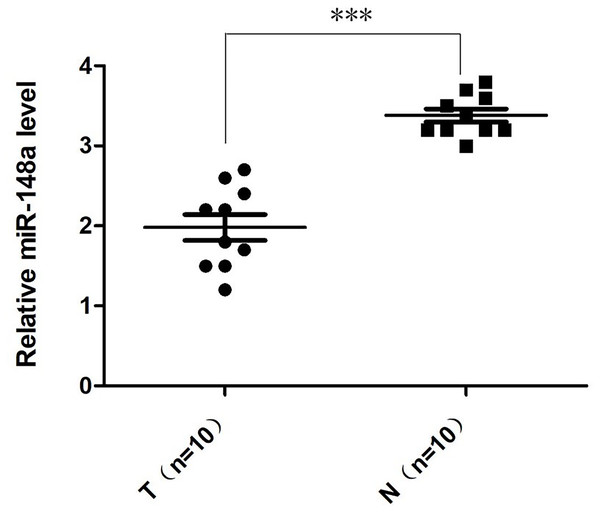
The relative quantification of miR-148a-3p expression in the bladder cancer tissues and adjacent tissues was shown in Fig. 1. According to the qRT-PCR, the expression level of miR‑148a-3p in bladder cancer tissues was significantly downregulated and lower than in adjacent tissues. There was a statistically significant difference between the groups (P < 0.001).
Figure 1: The relative quantification of miR-148a expression.
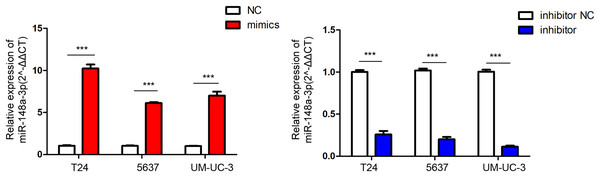
The relative quantification of miR-148a expression in the bladder cancer tissues (T) and adjacent tissues (N) by qRT-PCR. The expression level of miR-148a was significantly lower in bladder cancer tissues compared with that in adjacent normal tissues (***P < 0.001).The relative quantification of miR-148a-3p expression in the T24, 5,637, and UM-UC-3 cell lines after transfection are shown in Fig. 2. After the verification of qRT-PCR, the miR-148a-3p mimics and inhibitor were successfully transferred into different bladder tumor cell lines of T24, 5,637, and UM-UC-3.
Figure 2: The relative quantification of miR-148a-3p expression.
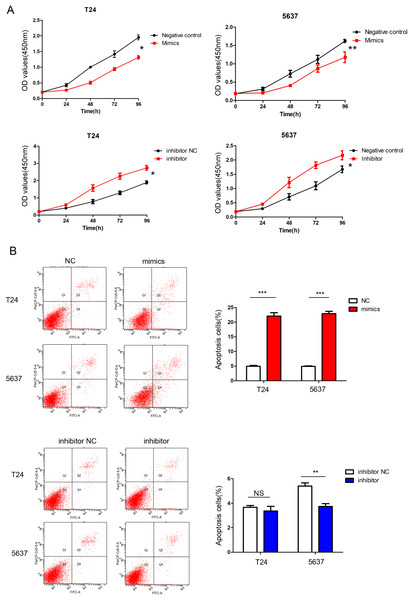
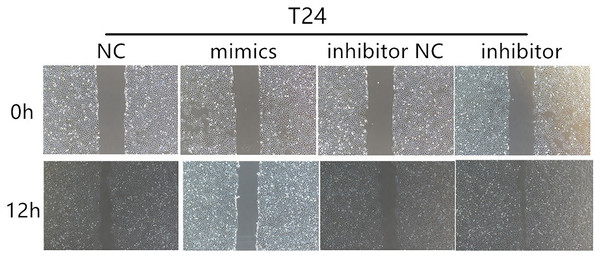
The relative quantification of miR-148a-3p expression in the T24, 5,637, and UM-UC-3 cell lines after transfection with the Lipofectamine 3,000 Reagent ***P < 0.001.According to the CCK-8 curve of T24 and 5,637 and the wound healing assay, we found that the intracellular transfer of miR-148a-3p mimics could inhibit cell proliferation, while the miR-148a-3p inhibitor could promote the proliferation of tumor cells when transferred into the cell. Moreover, overexpression of miR-148a-3p promoted cell apoptosis in the T24 and 5,637 cell lines. However, low expression of miR-148a-3p reduced the cell apoptosis only in the 5,637 cell lines (Fig. 3). The images of the wound healing assay were shown in Fig. 4.
Figure 3: miR-148a-3p inhibits cell proliferation and promotes cell apoptosis.
(A) CCK-8 curve of the T24 and 5,637 cell lines. Overexpression of miR-148a-3p inhibited cell proliferation as indicated by CCK-8 assays in the T24 and 5,637 cells. On the contrary, knockdown of miR-148a-3p promoted cell proliferation in the T24 and 5,637 cells. (B) Overexpression of miR-148a-3p promoted cell apoptosis in the T24 and 5,637 cell lines. However, low expression of miR-148a-3p reduced the cell apoptosis in only the 5,637 cell lines *P < 0.05, **P < 0.01, ***P < 0.001.Figure 4: The results of wound healing assay.
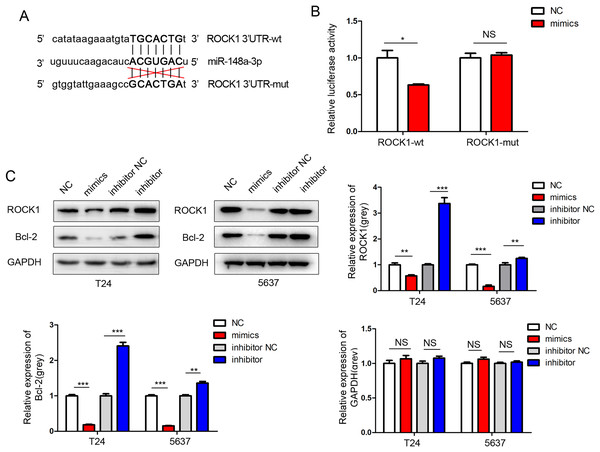
Wound healing assays for T24 cells after transfection with mimics and inhibitor of miR-148a-3p in 0 and 12 h.We performed a miRNA target gene prediction with the miRdb (http://mirdb.org/), miRbase (http://www.mirbase.org/), and Targetscan (http://www.targetscan.org/vert_71/) databases to explore the target gene of miR-148a-3p in bladder cancer. We found that there was a miR-148a-3p binding site in the 3′UTR region of ROCK-1 mRNA. Dual luciferase assays were performed to verify that ROCK-1 is a target of miR-148a-3p (Fig. 5A). As expected, the luciferase activity was significantly decreased with miR-148a-3p overexpression in the wild-type 3′UTR sequence of ROCK-1 compared with the mutant 3′UTR sequence of ROCK-1 (Fig. 5B), suggesting that ROCK-1 is a direct target of miR-148a-3p. Western blots showed that the expression level of ROCK-1 was downregulated after intracellular transfection of miR-148a-3p mimics. Conversely, after inhibition of miR-148a-3p, the expression level of ROCK-1 was upregulated (Fig. 5C). As overexpression of miR-148a-3p promoted cell apoptosis, we further explored the relationship between miR-148a-3p and Bcl-2. The expression of Bcl-2 decreased after transfection with miR-148a-3p mimics and increased after miR-148a-3p inhibitor transfection into the T24 and 5,637 cell lines, suggesting that miR-148a-3p may promote cell apoptosis by regulating the expression of Bcl-2 (Fig. 5C).
Figure 5: ROCK-1 is a direct target of miR-148a-3p.
(A) miR-148a-3p and its predicted binding sequence in the wild-type (wt) and mutant (mut) 3′UTRs of ROCK-1 mRNA. (B) Dual-luciferase assays showed that ROCK-1 is a target of miR-148a-3p. (C) Western blots showed that the expression levels of ROCK-1 and Bcl-2 were downregulated after miR-148a-3p overexpression. Conversely, the expression level of ROCK-1 was upregulated after miR-148a-3p knockdown *P < 0.05, **P < 0.01, ***P < 0.001.Discussion
Studies have shown that miRNAs could regulate the expression of tumor suppressors or oncogenes (Cheng et al., 2015; Png et al., 2011). Downregulation of miR-148a-3p is common in various cancers (Chen et al., 2010; Ueda et al., 2010), suggesting that miR-148a-3p significantly affects carcinogenesis and tumor progression.
The expression changes of miR-148a-3p, as a member of the miRNA family, are known to be related to the occurrence and development of bladder cancer (Wang et al., 2016; Xiang et al., 2019). It is well established that miRNAs perform their function by regulating the expression of a target gene. However, the downstream targets of this miRNA differ and it is unclear how miR-148a-3p modulates cellular responses in varying cellular contexts. Therefore, this study aimed to investigate the relationship between miR-148a-3p and the proliferation and migration of bladder tumor cells and to explore the specific mechanism of how miR-148a-3p regulates the biological behavior of bladder tumor cells.
In previous findings, our research team found that miR-148a-3p expression was decreased in bladder cancer specimens (Ma et al., 2016). In the current study, we validated this result. According to the qRT-PCR, the expression level of miR‑148a-3p in bladder cancer tissues was significantly downregulated and lower than in adjacent tissues. Initially, the belief was that the decrease of miR-148a expression in cancer tissues inhibited its anti-cancer effects, which leads to the occurrence and development of bladder cancer.
Then, we focused on the role of miR-148a-3p on the pathogenic mechanism of bladder cancer cells; including invasion, proliferation, and apoptosis. We selected three bladder tumor cell lines, T24, 5,637 and UM-UC-3, to investigate miR-148-3p’s regulatory mechanism. miR-148a-3p mimics and inhibitor were successfully transferred into different bladder tumor cell lines of T24, 5,637, and UM-UC-3 and verified by qRT-PCR. In this study, the expression levels of miR-148a-3p were artificially regulated with the miR-148a-3p mimics and the miR-148a-3p inhibitor. Our gain- and loss-of function experiments demonstrated that the miR-148a-3p inhibitor led to the increase of invaded bladder cancer cells, whereas the miR-148a-3p mimics inhibited the invasion ability, and the difference of the expression level was statistically significant (P < 0.001). The CCK-8 curve of the T24 and 5,637 cells and the wound healing assay showed that the intracellular transfer of miR-148a-3p mimics could inhibit cell proliferation. However, the miR-148a-3p inhibitor could promote the proliferation of tumor cells when transferred into the cell. Overexpression of miR-148a-3p promoted cell apoptosis in the T24 and 5,637 cell lines, which suggested that miR-148a-3p might act as a tumor suppressor by inducing cell apoptosis in bladder cancer.
We performed a miRNA target gene prediction with the miRdb (http://mirdb.org/), miRbase (http://www.mirbase.org/), and Targetscan (http://www.targetscan.org/vert_71/) databases and found that there was a miR-148a-3p binding site in the 3′UTR region of ROCK-1 mRNA. After that, dual luciferase assays were performed and verified that ROCK-1 is a direct target of miR-148a-3p. The expression level of ROCK-1 was downregulated after intracellular transfection of miR-148a-3p mimics with western blotting. Conversely, after inhibition of miR-148a-3p, the expression level of ROCK-1 was upregulated. As we found that overexpression of miR-148a-3p promoted cell apoptosis, we further explored the relationship between miR-148a-3p and Bcl-2, which played an important role in inhibiting cell apoptosis. The expression of Bcl-2 decreased after miR-148a-3p mimics transfection and increased after miR-148a-3p inhibitor transfection into the T24 and 5,637 cell lines, suggesting that miR-148a-3p may promote cell apoptosis by regulating the expression of Bcl-2, which further verified that miR-148a-3p acts as a tumor suppressor by inducing cell apoptosis in bladder cancer.
Considering that miR-148a-3p inhibits the progression of bladder cancer, it can be used for miRNA-based therapy for bladder cancer patients. miRNAs are particularly abundant in exosomes (Izumi et al., 2015), moreover, exosomes also protect miRNAs from RNase degradation (Koga et al., 2011) These provide a novel method for miRNA-based therapy (Del Pozo-Acebo et al., 2021). Therefore, the combined application of miR-148a-3p and exosomes for extracellular miRNA delivery has considerable development prospects.
In conclusion, our research verified that miR-148a-3p functions as a tumor-suppressing miRNA by directly targeting ROCK-1’s mRNA and inducing cell apoptosis by regulating the expression of Bcl-2. However, the deeper mechanism of this regulatory relationship needs further study.